Asymmetric Synthesis of Diamine Derivatives via Sequential Palladium and Rhodium Catalysis
Barry M. Trost, Sushant Malhotra, David E. Olson, Autumn Maruniak, and J. Du Bois
Journal of the American Chemical Society,
2009, 4190-4191; 10.1021/ja809697p
January 2009
Polysubstituted, chiral diamines are key structural motifs in a wide variety of Natural Product and pharmaceutical target skeletons. This collaborative report from the labs of Prof. Du Bois and Prof. Trost, both based at Stanford, combines two powerful methodologies to significantly streamline and expand the scope of substituted diamine synthesis.
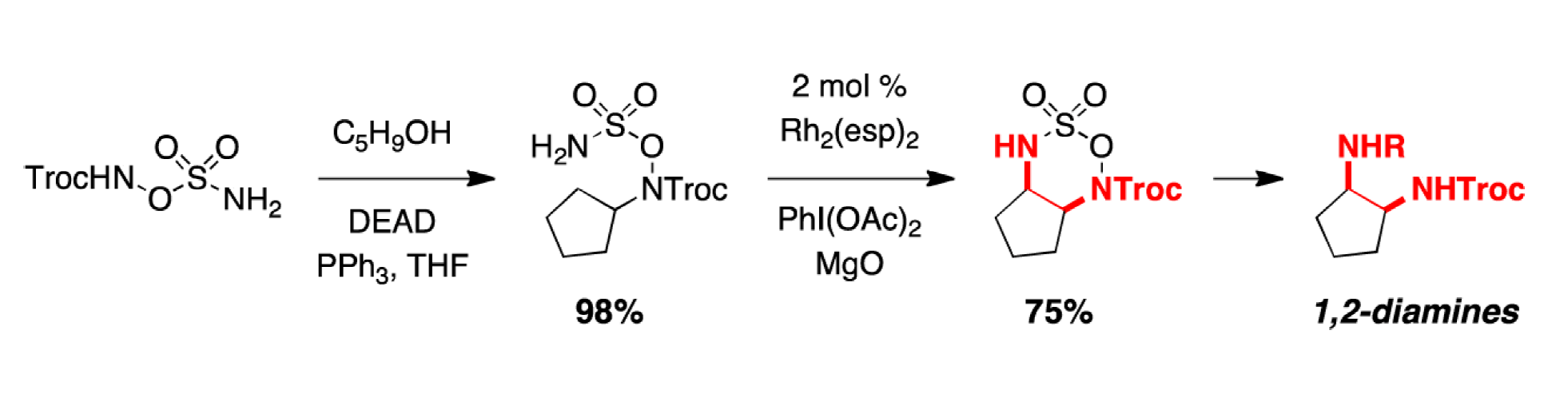
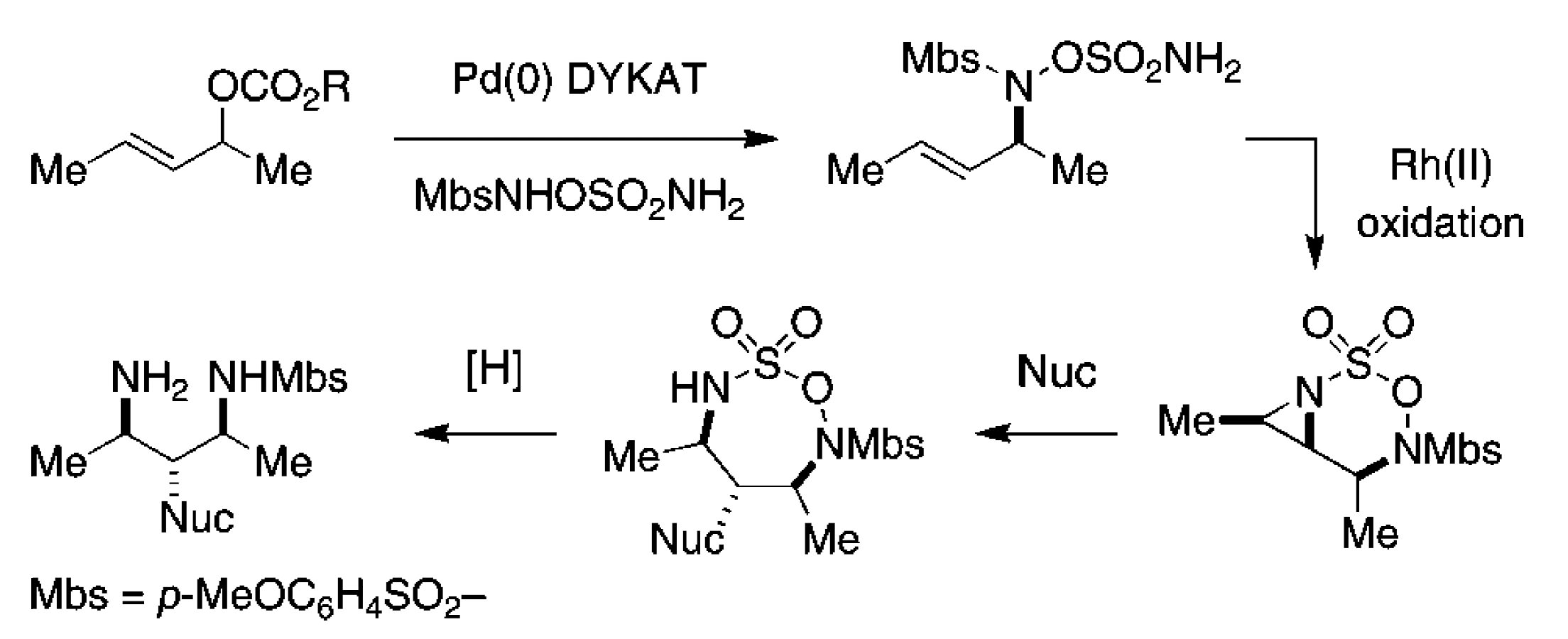
The first step employs the Palladium-catalyzed Dynamic Kinetic Asymmetric Transformation (DYKAT's) developed by the Trost group to convert racemic allylic ethers to chiral allyl amines. This substrate could then be effectively converted to the corresponding aziridine using the rhodium(II)-catalyzed C–H amination reaction developed by the Du Bois group. Ring opening of these systems with a variety of nucleophiles completed the rapid access to highliy substituted, stereochemically defined architectures.
This work represents a powerful new methodology for the synthesis of complex stereodefined molecular motifs based on C–H functionalization.