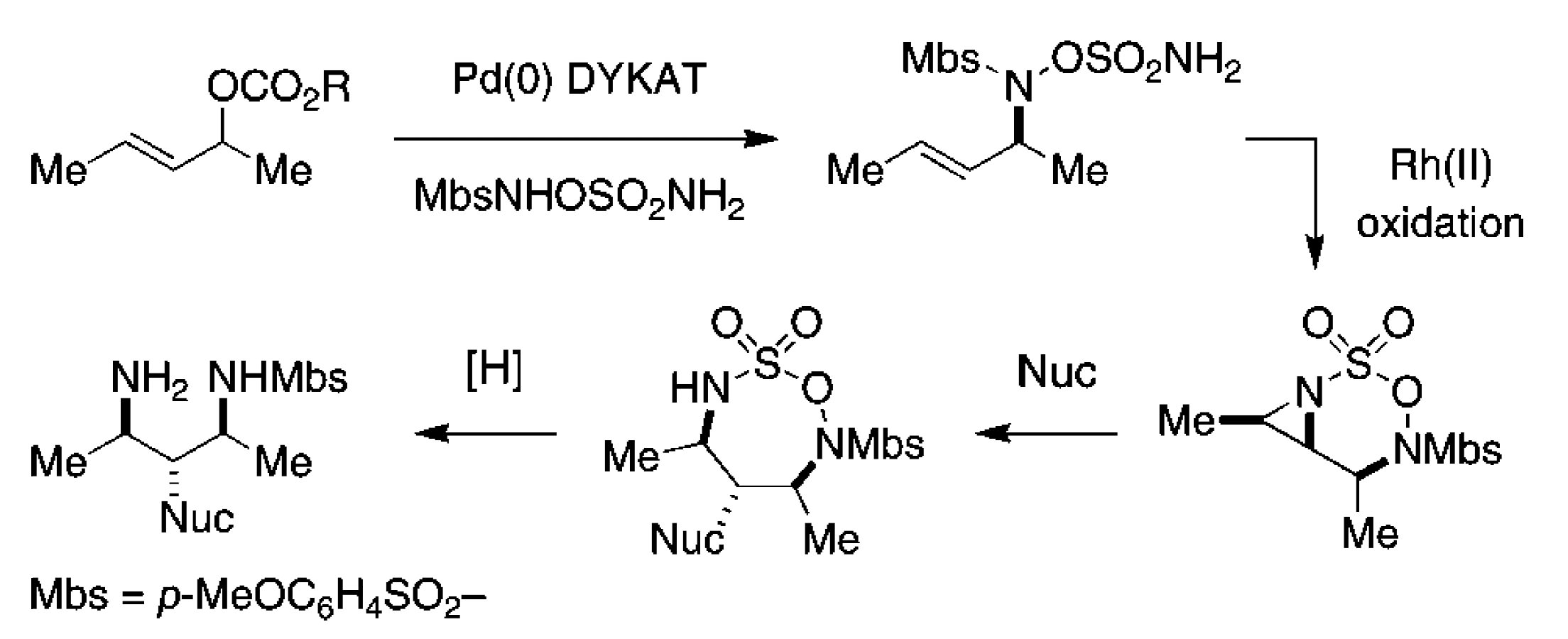
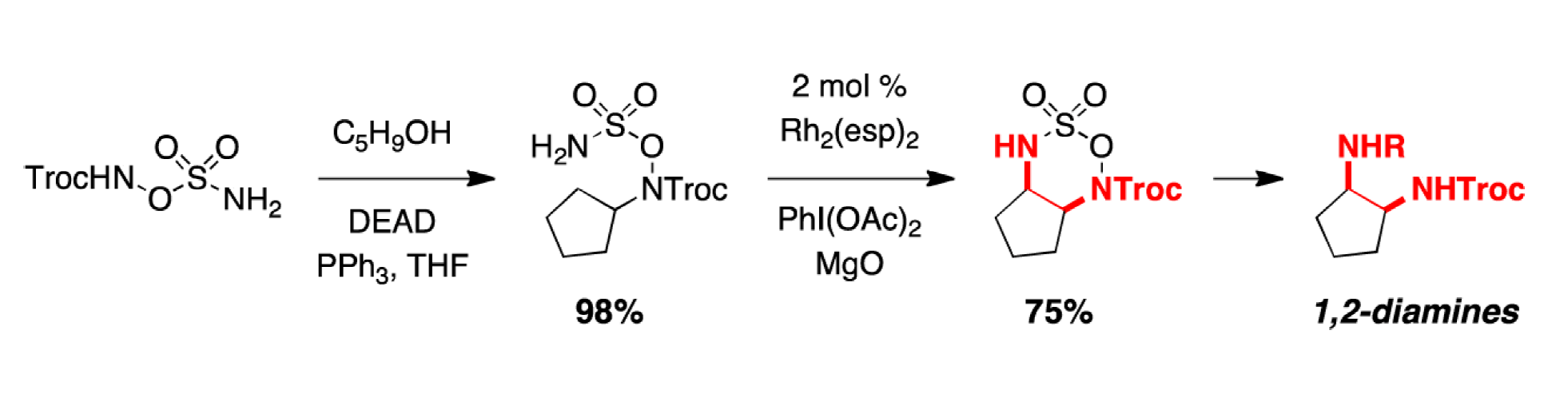
Synthesis of Differentially Substituted 1,2-Diamines through Advances in C–H Amination Technology
Olsen, D. E., Roberts, D. A., Du Bois, J.
Organic Letters,
2012, 6174-6177; 10.1021/ol302895f
10/2012
A general, high yielding method for the synthesis of 1,2-diamine derivatives is described that capitalizes on selective, rhodium-catalyzed C–H insertion of hydroxylamine-based sulfamate esters. The resulting Troc-protected oxathiadiazinane heterocycles are easily modified and can be reduced under the mild action of NaI to afford differentially substituted diamine products. This technology offers a number of salient improvements over related C–H and π-bond amination tactics for diamine synthesis.