Bystanding F+ Oxidants Enable Selective Reductive Elimination from High-Valent Metal Centers in Catalysis
Keary M. Engle, Tian-Sheng Mei, Xisheng Wang, Jin-Quan Yu
Angew. Chem. Int. Ed.,
2011, 50, 7, 1478-1491; 10.1002/anie.201005142
01/2011
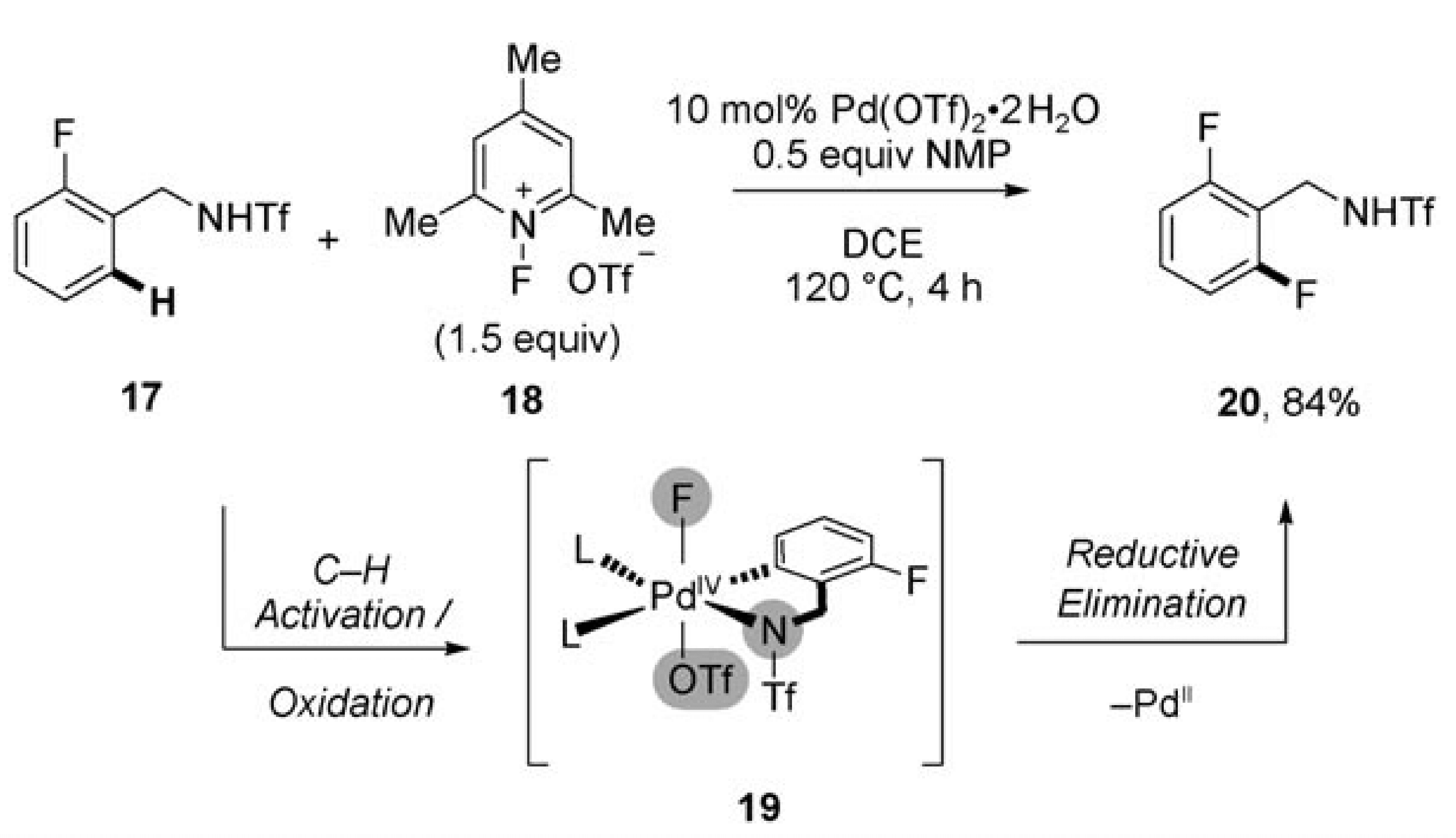
Transition-metal catalysis has become a central tenant in the logic of synthetic organic chemistry. In the development of these reactions much optimization and a thorough understanding of the mechanistic details has been required. This review discusses one particular step of the catalytic cycle; reductive elimination.
In circumstances where this step has proved challenging one strategy adopted has been to use an external two-electron oxidant to promote the oxidation of the metal center and complete the catalytic cycle. However, many of commonly suitable oxidants for these transformations incorporate a heteroatom, such as oxygen, nitrogen or a halogen, which can interfere with the reaction and significantly impact efficacy and conversion.
This review emphasizes the use of F+ oxidants which act as bystanders and rarely engage in undesired reactions. The key example, which elegantly highlights the future potential of this approach, is a palladium(II)-catalyzed C–H Functionalization.