The Combined C-H Functionalization/Cope Rearrangement: Discovery and Applications in Organic Synthesis
Davies, H. M. L., Lian, Y.
Acc. Chem. Res.,
2012, 923-935; 10.1021/ar300013t
01/2012
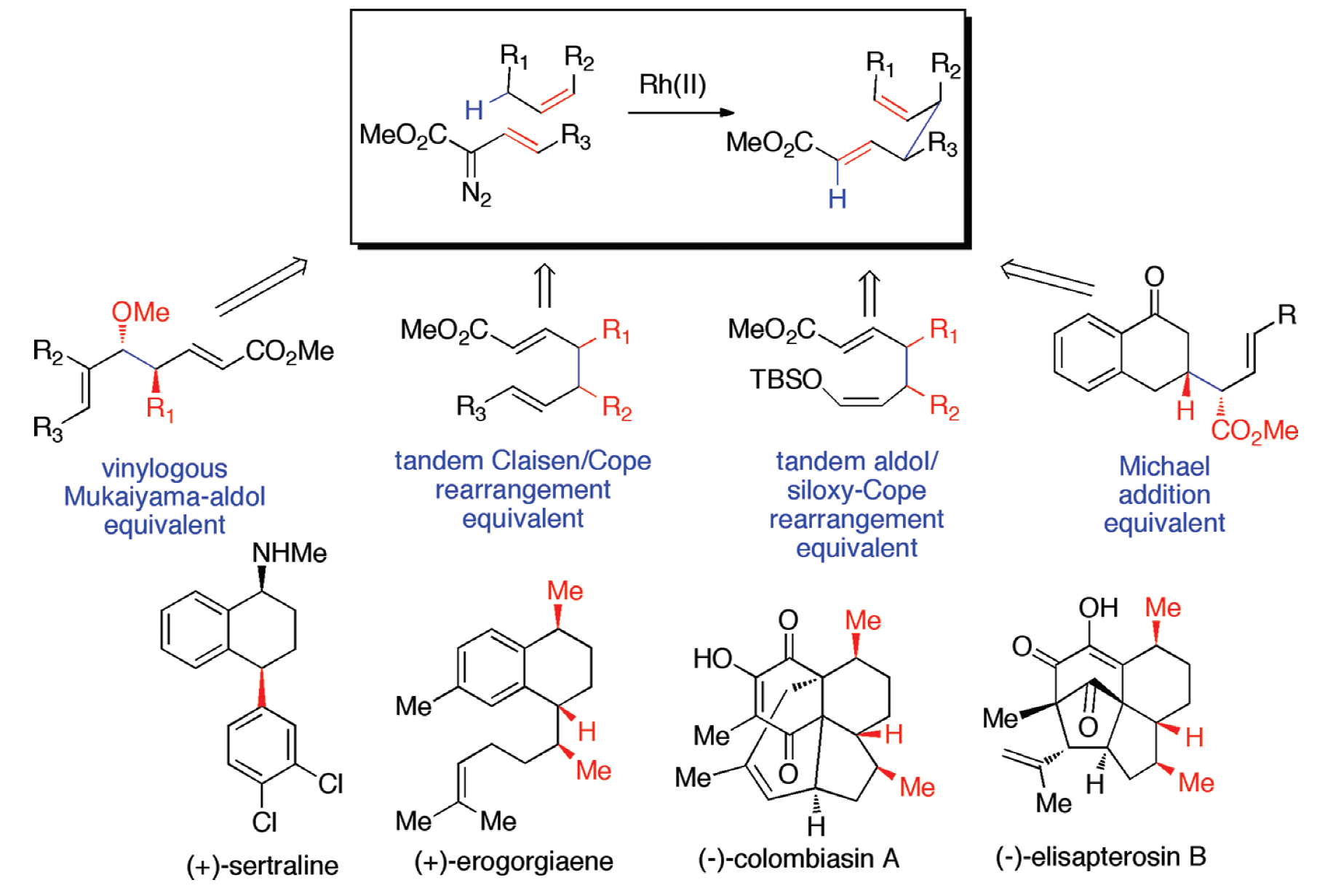
The development of methods for the stereoselective functionalization of sp3 C–H bonds is a challenging undertaking. This Account describes the scope of the combined C–H functionalization/Cope rearrangement (CHCR), a reaction that occurs between rhodium-stabilized vinylcarbenoids and substrates containing allylic C–H bonds.
The CHCR reaction has broad applications in organic synthesis. In some new protocols, the CHCR reaction acts as a surrogate to some of the classic synthetic strategies in organic chemistry. The CHCR reaction has served as a synthetic equivalent of the Michael reaction, the vinylogous Mukaiyama aldol reaction, the tandem Claisen rearrangement/Cope rearrangement, and the tandem aldol reaction/siloxy-Cope rearrangement. In all of these cases, the products are generated with very high diastereocontrol.