Catalytic C–H bond silylation of aromatic heterocycles
A. A Toutov, W.-B. Liu, K. N Betz, B. M Stoltz and R. H Grubbs
Nature Protocols
2015, 10, 1897-1903; 10.1038/nprot.2015.118
10/2015
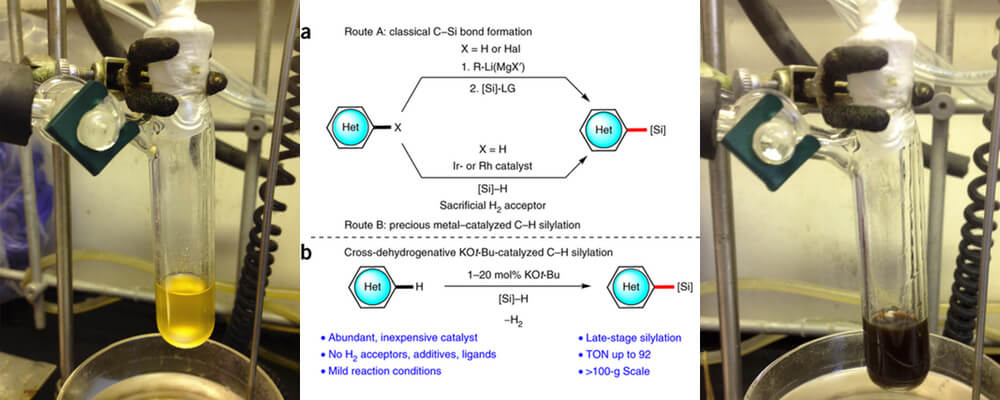
The Stoltz and Grubbs group have previously disclosed the development of a novel one-pot catalytic C–H bond silylation chemistry, with a particular focus on heterocycles found in pharmaceutical targets.
This paper in Nature Protocols expands upon this work, not only by providing a detailed and general step-wise procedure for this earth-abundant element catalyzed transformation but also extending the scope of this transformation to more complex systems. It is demonstrated that pharmaceuticals such as Ticlopidine and Thenalidine can be readily functionalized, important when considering potential generation of sila-therapeutics.
These investigations are part of a continuing effort in the Center to understand the mechanism behind this catalytic transformation and application of it to novel transformations.

