A potassium tert-butoxide and hydrosilane system for ultra-deep desulfurization of fuels
A. A. Toutov, M. Salata, A. Fedorov, Y.-F. Yang, Y. Liang, R. Cariou, K. N. Betz, E. P. A. Couzijn, J. W. Shabaker, K. N. Houk and R. H. Grubbs
Nature Energy
2017, 2, 17008; DOI: 10.1038/nenergy.2017.8
02/2017
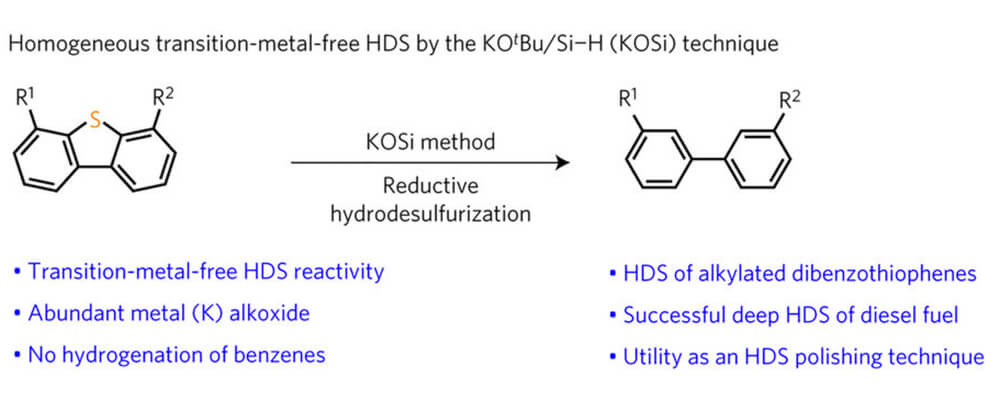
Hydrodesulfurization (HDS) is the process by which sulfur-containing impurities are removed from petroleum streams, typically using a heterogeneous, sulfided transition metal catalyst under high H2 pressures and temperatures. Although generally effective, a major obstacle that remains is the desulfurization of highly refractory sulfur-containing heterocycles, such as 4,6-dimethyldibenzothiophene (4,6-Me2DBT), which are naturally occurring in fossil fuels.
Homogeneous HDS strategies using well-defined molecular catalysts have been designed to target these recalcitrant S-heterocycles; however, the formation of stable transition metal sulfide complexes following C–S bond activation has largely prevented catalytic turnover. Here we show that a robust potassium (K) alkoxide (O)/hydrosilane (Si)-based (‘KOSi’) system efficiently desulfurizes refractory sulfur heterocycles. Subjecting sulfur-rich diesel (that is, [S] ∼ 10,000 ppm) to KOSi conditions results in a fuel with [S] ∼ 2 ppm, surpassing ambitious future governmental regulatory goals set for fuel sulfur content in all countries.
Related Content
-
12/2016
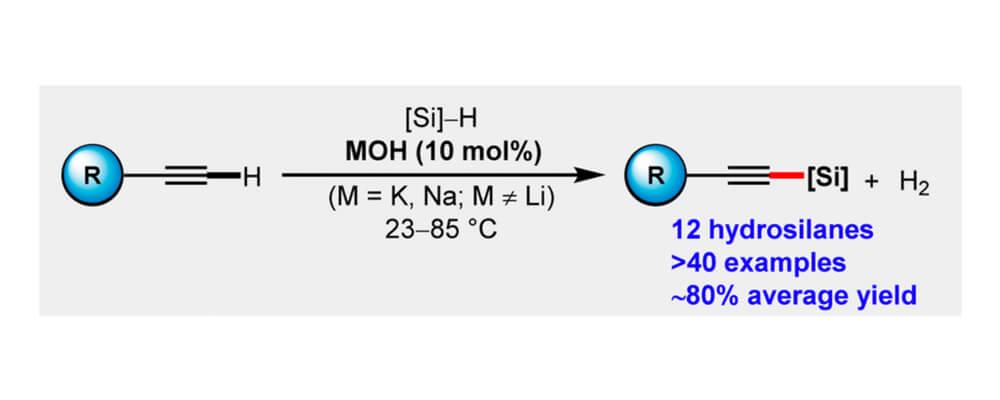
Alkali Metal-Hydroxide-Catalyzed C(sp)–H Bond silylation
RESEARCH
-
11/2016
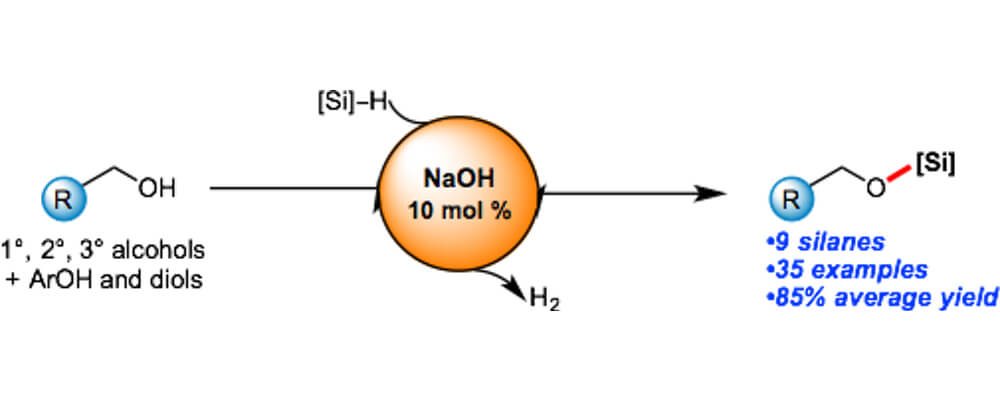
Sodium Hydroxide Catalyzed Dehydrocoupling of Alcohols with Hydrosilanes
RESEARCH
-
08/2016
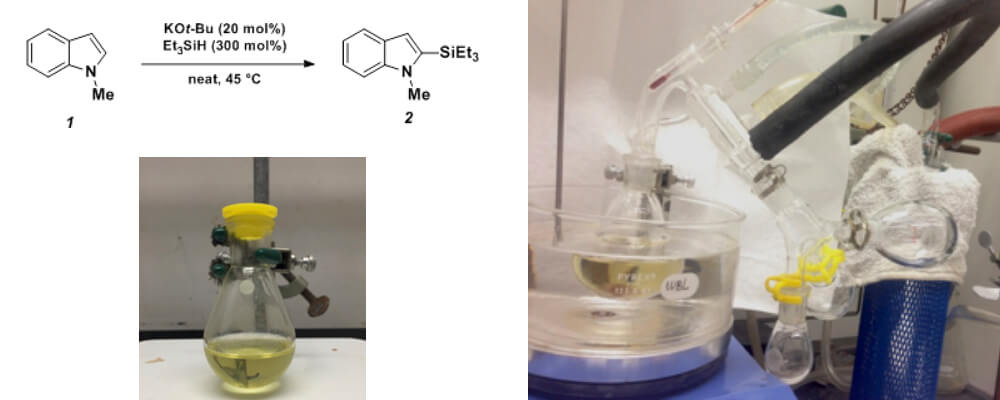
Potassium tert-Butoxide-Catalyzed Dehydrogenative Cross-Coupling of Heteroarenes with Hydrosilanes
RESEARCH
-
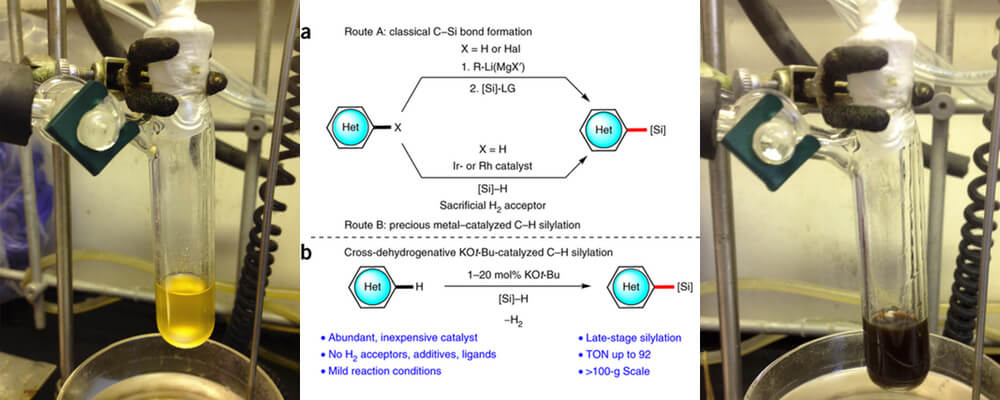
10/2015
Catalytic C–H bond silylation of aromatic heterocycles
RESEARCH
-

01/2015
Silylation of C–H bonds in aromatic heterocycles by an Earth-abundant metal catalyst
RESEARCH