Selective C–H bond functionalization using repurposed or artificial metalloenzymes
David M Upp, Jared C Lewis
Curr. Opin. Chem. Biol.
2017, 37, 48-56; DOI: 10.1016/j.cbpa.2016.12.027
02/2017
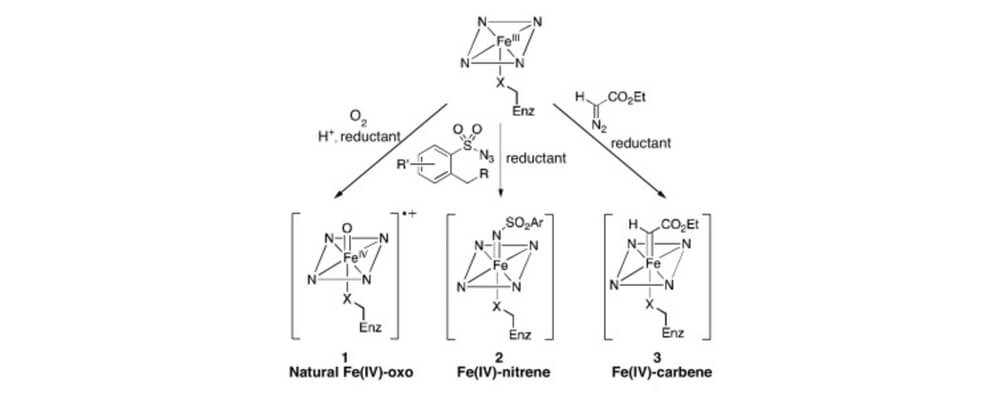
Catalytic C–H bond functionalization has become an important tool for organic synthesis. Metalloenzymes offer a solution to one of the foremost challenges in this field, site-selective C–H functionalization, but they are only capable of catalyzing a subset of the Csingle bondH functionalization reactions known to small molecule catalysts.
To overcome this limitation, metalloenzymes have been repurposed by exploiting the reactivity of their native cofactors toward substrates not found in nature. Additionally, new reactivity has been accessed by incorporating synthetic metal cofactors into protein scaffolds to form artificial metalloenzymes. The selectivity and activity of these catalysts has been tuned using directed evolution. This review covers the recent progress in developing and optimizing both repurposed and artificial metalloenzymes as catalysts for selective C–H bond functionalization.
Related Content
-
02/2016
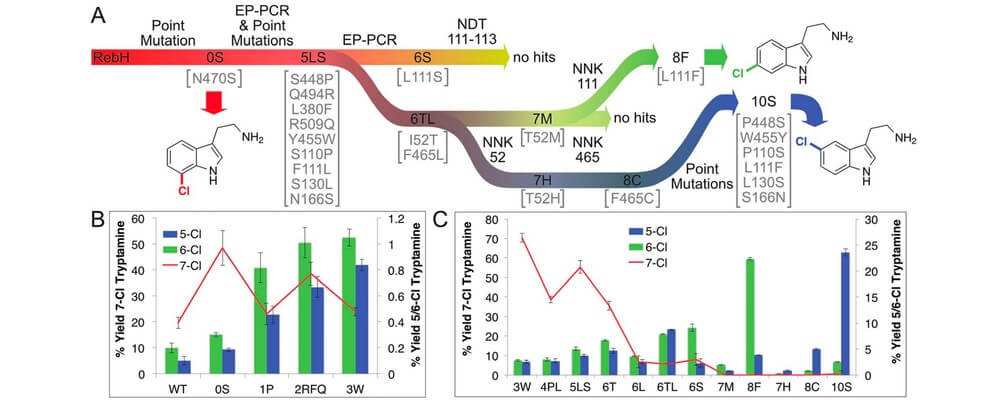
Directed evolution of RebH for catalyst-controlled halogenation of indole C–H bonds
RESEARCH
-

07/2015
Engineering a dirhodium artificial metalloenzyme for selective olefin cyclopropanation
RESEARCH
-
07/2014
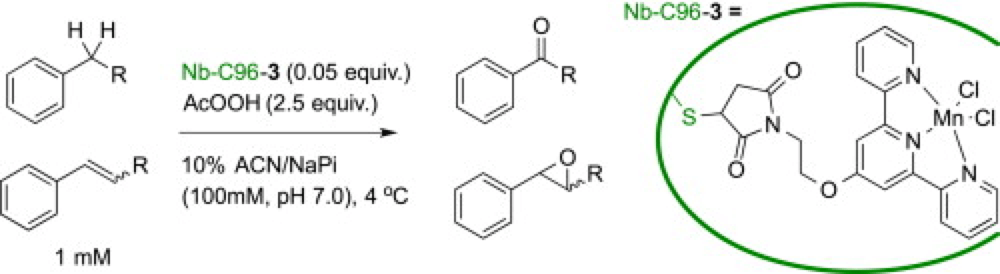
Manganese terpyridine artificial metalloenzymes for benzylic oxygenation and olefin epoxidation
RESEARCH
-
12/2013
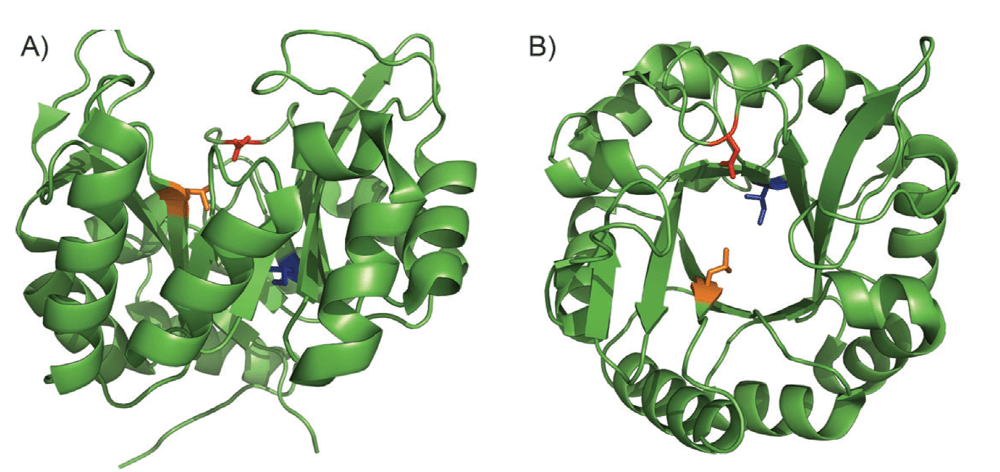
A General Method for Artificial Metalloenzyme Formation through Strain-Promoted Azide–Alkyne Cycloaddition
RESEARCH
-
10/2013
Artificial Metalloenzymes and Metallopeptide Catalysts for Organic Synthesis
RESEARCH