Decoding cyclase-dependent assembly of hapalindole and fischerindole alkaloids
Shasha Li, Andrew N Lowell, Sean A Newmister, Fengan Yu, Robert M Williams and David H Sherman
Nature, Chemical Biology,
2017, 13, 467-469; DOI: 10.1038/nchembio.2327
03/2017
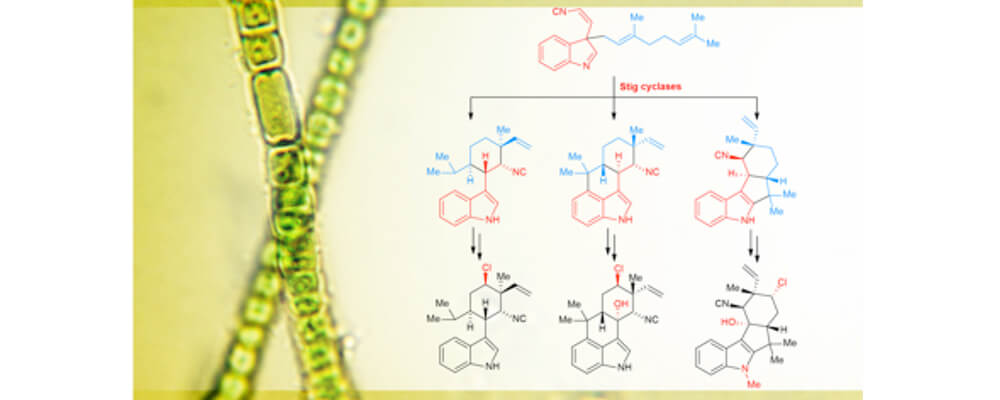
Hapalindoles are cyanobacterial alkaloids well-known for their unique pharmacological profiles and complex chiral structures. The most notable structural feature of this family is their diverse polycyclic ring system that show a multiplicity of stereo- and regiochemical C-C bond connections. Our research focuses on understanding the biosynthetic pathway toward the purpose of developing chemoenzymatic and bio-mimic inspired syntheses to produce new variations for drug discovery.
In this report, we identified and elucidated the class of Stig cyclases that catalyzes enantioselective tri- and tetracyclic core formation via a Cope rearrangement and C-C bond-forming cascade. This understanding is the first step toward enabling functionalization of these complex scaffolds, following studies will explore the molecular basis for late-stage tailoring through C-H functionalization.
Author: Shasha Li