Key mechanistic insights into the intramolecular C-H bond amination and double bond aziridination in sulfamate esters catalyzed by dirhodium tetracarboxylate complexes
Adrián Varela-Álvarez, Brandon E.Haines, Djamaladdin G.Musaev
J. Organometallic Chem.,
2018, 867, 183; DOI:10.1016/j.jorganchem.2017.12.013
01/2018
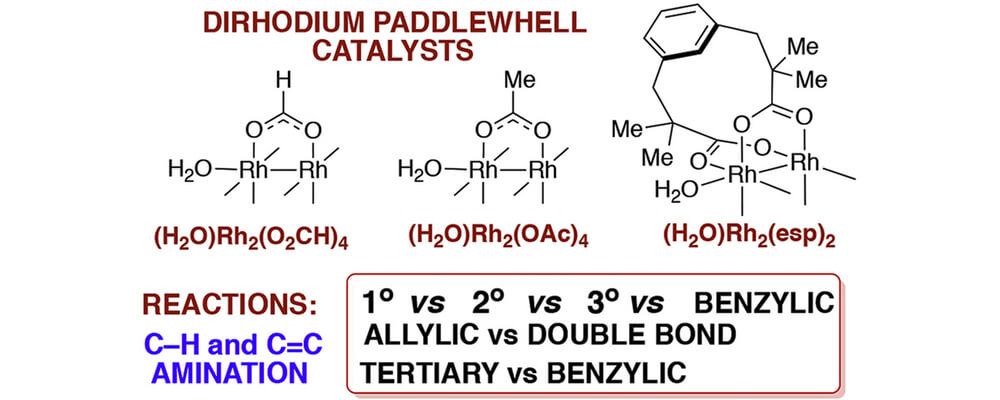
We study the mechanisms of the dirhodium paddlewheel complexes catalyzed intramolecular C-H amination and olefin aziridination of a variety of sulfamate esters. Particular emphasis is placed on the mechanism and factors governing amination of primary, secondary, tertiary and benzylic C-H bonds, the competition between tertiary and benzylic C-H amination, and the competition between allylic C-H amination and olefin aziridination. Active catalytic species are found to be triplet state dirhodium-nitrene complexes with the Rh–Rh single bond and Rh-N double bond. It is shown that the C-H amination proceeds via triplet-to-singlet surface crossing and singlet state concerted C-H insertion mechanism. With the allylic substrate, the competing C=C double bond aziridination follows a stepwise pathway involving the radical intermediate formation and radical coupling steps.