Inverting Steric Effects: Using “Attractive” Noncovalent Interactions To Direct Silver-Catalyzed Nitrene Transfer
Minxue Huang, Tzuhsiung Yang, Jonathan D. Paretsky, John F. Berry, and Jennifer M. Schomaker
J. Am. Chem. Soc.
2017, 139 (48), pp 17376–17386; DOI: 10.1021/jacs.7b07619
11/2017
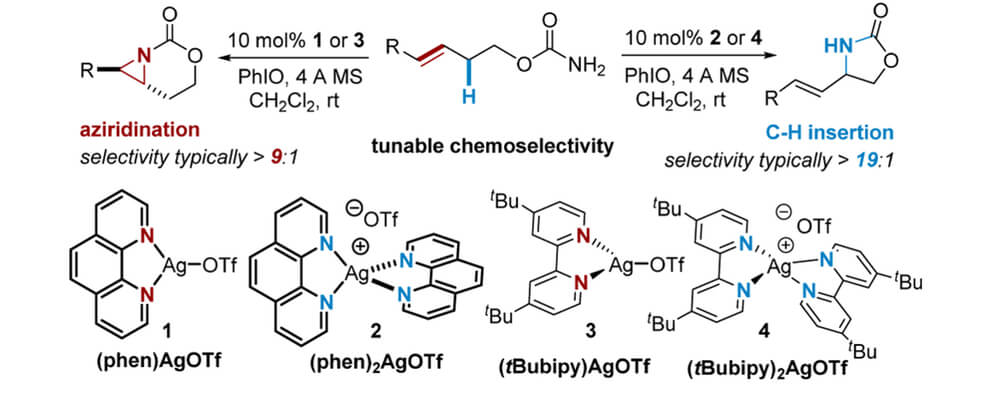
Nitrene transfer (NT) reactions represent powerful and direct methods to convert C−H bonds into amine groups that are prevalent in many commodity chemicals and pharmaceuticals. The importance of the C−N bond has stimulated the development of numerous transition-metal complexes to effect chemo-, regio-, and diastereoselective NT. An ongoing challenge is to understand how subtle interactions between catalyst and substrate influence the site-selectivity of the C−H amination event.
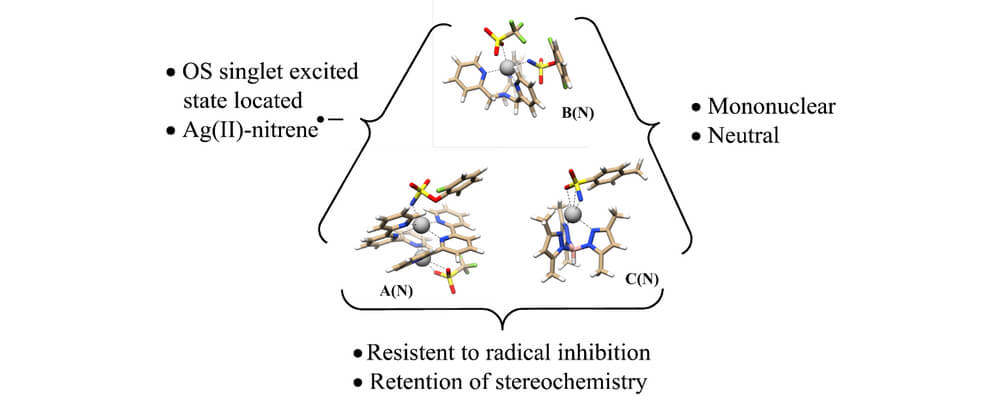
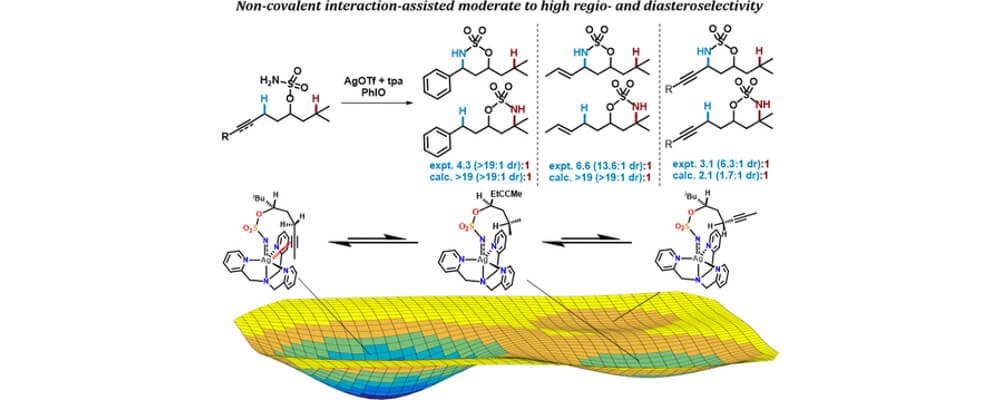
In this work, the Berry and Schomaker groups explore the underlying reasons why Ag(tpa)OTf (tpa = tris(pyridylmethyl)amine) prefers to activate α-conjugated C−H bonds over 3° alkyl C(sp3)−H bonds and apply these insights to reaction optimization and catalyst design. Experimental results suggest possible roles of noncovalent interactions (NCIs) in directing the NT; computational studies support the involvement of π···π and Ag···π interactions between catalyst and substrate, primarily by lowering the energy of the directed transition state and reaction conformers. A simple Hess’s law relationship can be employed to predict selectivities for new substrates containing competing NCIs. The insights presented herein are poised to inspire the design of other catalyst-controlled C−H functionalization reactions.