Comparison of Reactivity and Enantioselectivity between Chiral Bimetallic Catalysts: Bismuth–Rhodium- and Dirhodium-Catalyzed Carbene Chemistry
Zhi Ren, Travis L. Sunderland, Cecilia Tortoreto, Tzuhsiung Yang, John F. Berry, Djamaladdin G. Musaev, and Huw M. L. Davies
ACS Catalysis,
2018, 8, (11), 10676-10682; DOI:10.1021/acscatal.8b03054
09/2018
Catalysis involving dirhodium paddlewheel complexes has been demonstrated as a widely used method in organic synthesis. The development of the ligands has been the focus to achieve outstanding selectivity and reactivity. However, the modification of the bimetallic core has been rarely studied.
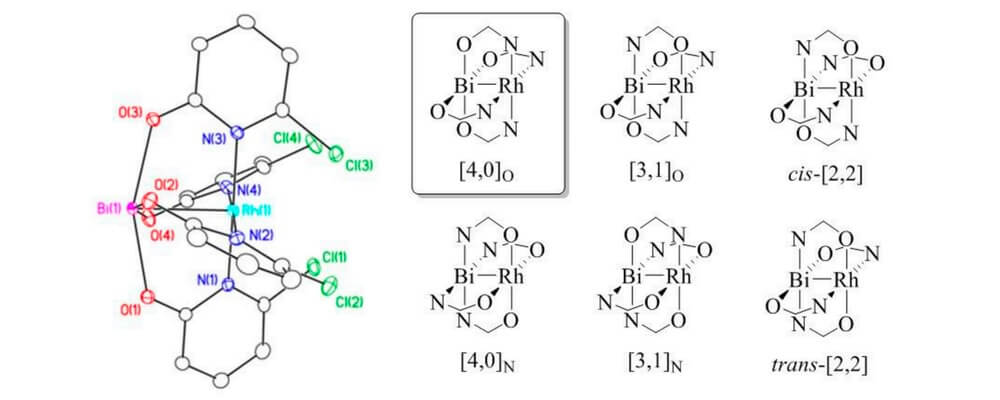
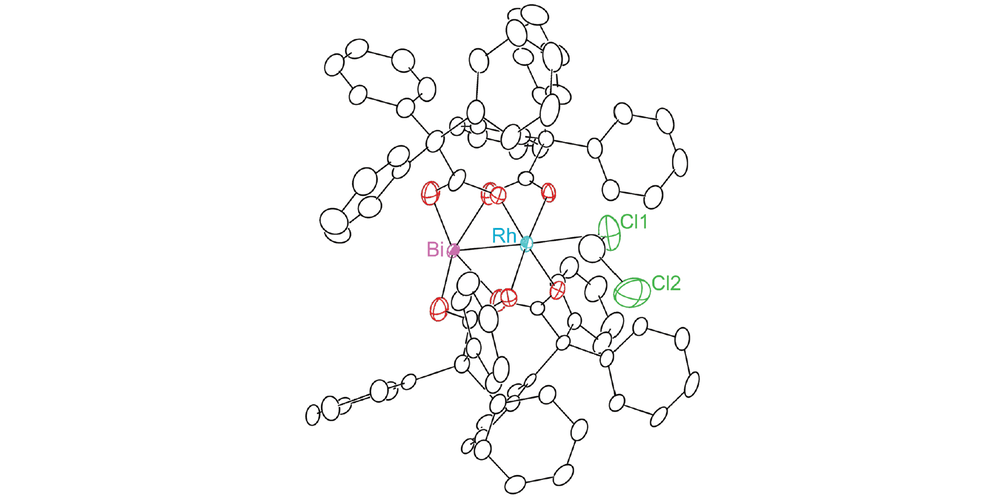
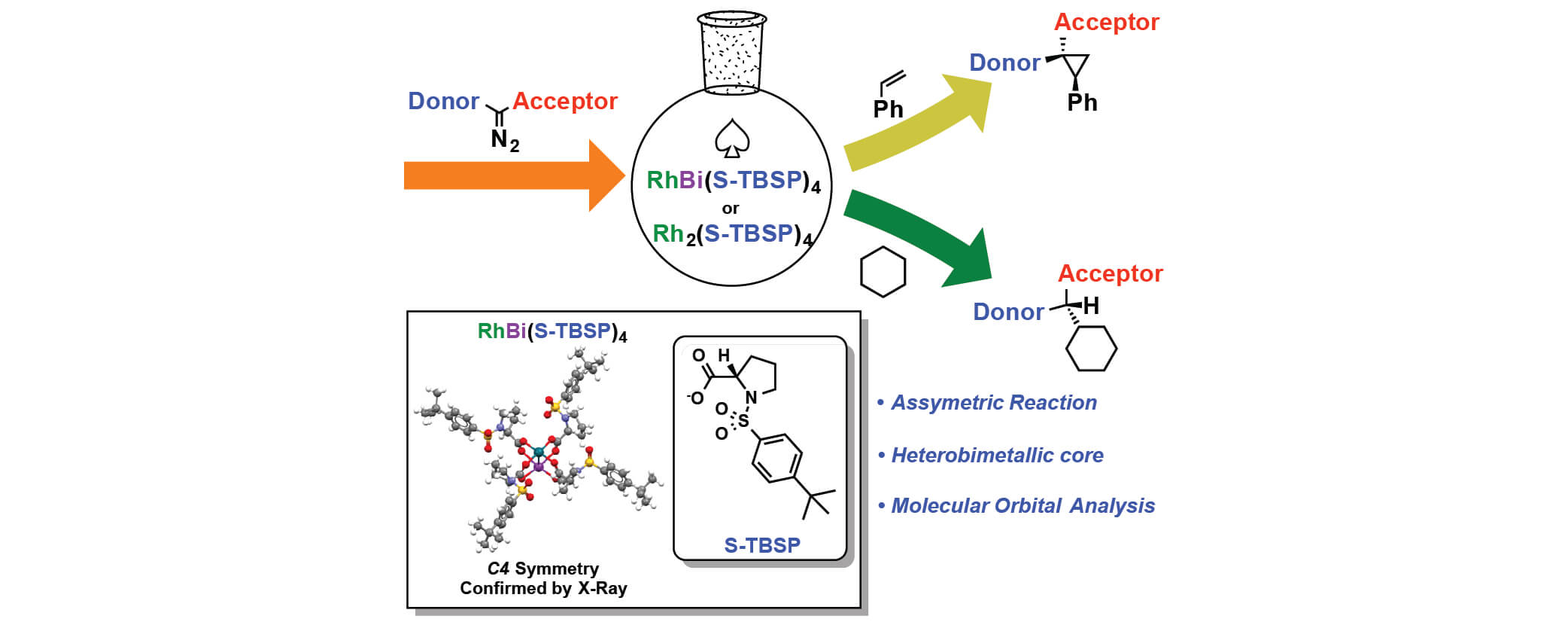
In this paper, Davies group has collaborated with Berry group and Musaev group to study the influence of replacing a Rh atom to Bi atom in donor/acceptor carbene transformations. Both complexes can effectively catalyze the reaction in good yields and enantiomeric excess for cyclopropanation and C-H functionalization reaction. But the rate of RhBi catalyzed reaction is much slower than the dirhodium catalyst, and the dirhodium carbene can react with a wider range of substrates. To further understand the similarities and differences between the two metal carbenes, the mechanistic and computational studies were conducted.
In this collaboration, the RhBi catalyst was prepared in Berry group with the chiral ligand from Davies group. The cyclopropanation and C-H functionalization reactions were conducted in Davies lab. The further mechanistic and computational discussions between the three groups led to more interesting studies for understanding the effect of replacing a Rh atom.