Understanding Flavin-Dependent Halogenase Reactivity via Substrate Activity Profiling
Mary C. Andorfer, Jonathan E. Grob, Christine E. Hajdin, Julia R. Chael, Piro Siuti, Jeremiah Lilly, Kian L. Tan, and Jared C. Lewis
ACS Catalysis,
2017, 7, (3), 1897-1904; DOI: 10.1021/acscatal.6b02707
01/2017
Halogenated aromatic compounds are abundant in nature and often exhibit unique and useful biological and synthetic properties. Aromatic halogenation is usually achieved through electrophilic aromatic substitution, and thus site selectivity of halogenation reactions is frequently determined by substrate electronic properties (i.e. which sites are the most electronically activated).
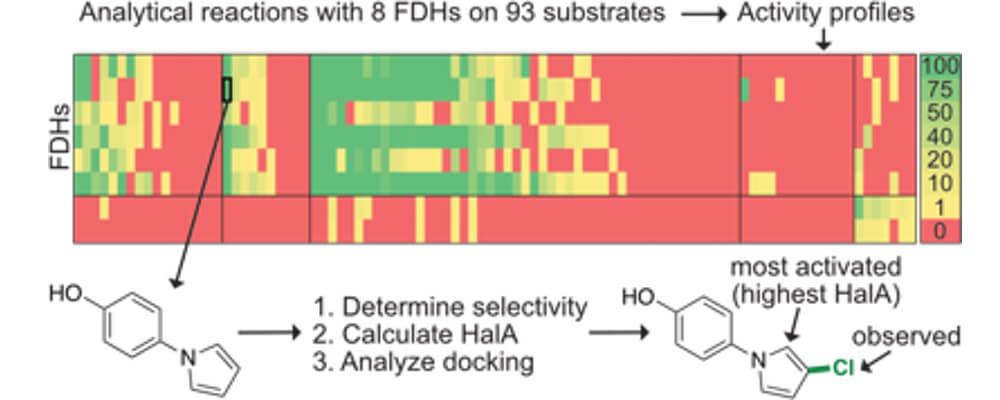
Flavin-dependent halogenases (FDHs) are capable of overriding substrate bias to chlorinate and brominate sites other than the most activated. In this study, the Lewis Group, in collaboration with our partners at the Novartis Institutes for Biomedical Research, explored the scope and selectivity of four wild-type FDHs and four engineered FDHs on 93 low molecular weight compounds, constituting the largest, most diverse panel of substrates analyzed to date.
Interestingly, FDHs were found to not only halogenate the majority of substrates tested, but were found to site-selectivity halogenate many of these substrates as well, most of which differed substantially from native FDH substrates. By analyzing these data in combination with computational studies, we were able to learn about trends in FDH substrate specificity, selectivity, functional group tolerance, and substrate binding.