Synthesis of Donor/Acceptor-Substituted Diazo Compounds in Flow and Their Application in Enantioselective Dirhodium-Catalyzed Cyclopropanation and C–H Functionalization
Daniel Rackl, Chun-Jae Yoo, Christopher W. Jones, and Huw M. L. Davies
Organic Letters,
2017, 19 (12), pp 3055–3058; DOI:10.1021/acs.orglett.7b01073
06/2017
This collaborative report from the Davies and Jones Groups build on previous work within the Center focused on the development of novel flow reactor systems to harness the potential of carbene C–H insertion transformations.
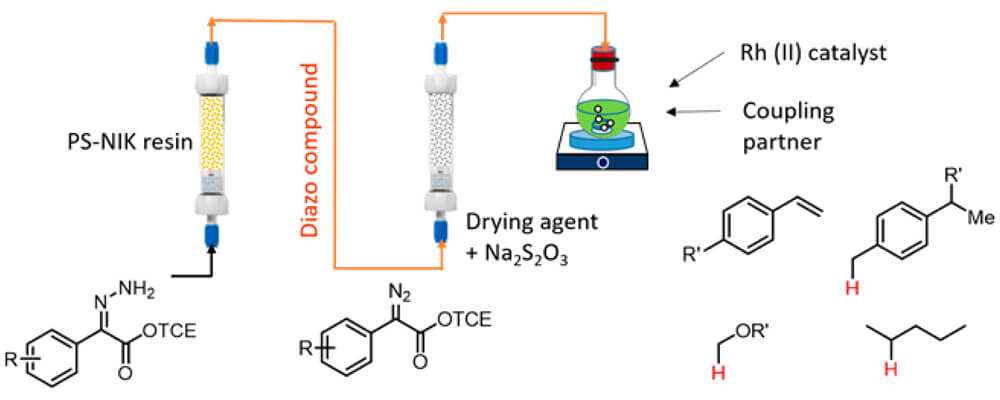
A tandem reaction system has been developed for the preparation of donor/acceptor-substituted diazo compounds in continuous flow coupled to dirhodium-catalyzed C–H functionalization or cyclopropanation. Hydrazones were oxidized in flow by solid-supported N-iodo-p-toluenesulfonamide potassium salt (PS-SO2NIK) to generate the diazo compounds, which were then purified by passing through a column of molecular sieves/sodium thiosulfate.
Related Content
-

06/2017
Synthesis of D/A-Substituted Diazo Compounds in Flow and Their Application in Enantioselective Dirhodium-Catalyzed Cyclopropanation and C–H Functionalization
RESEARCH
-
04/2015
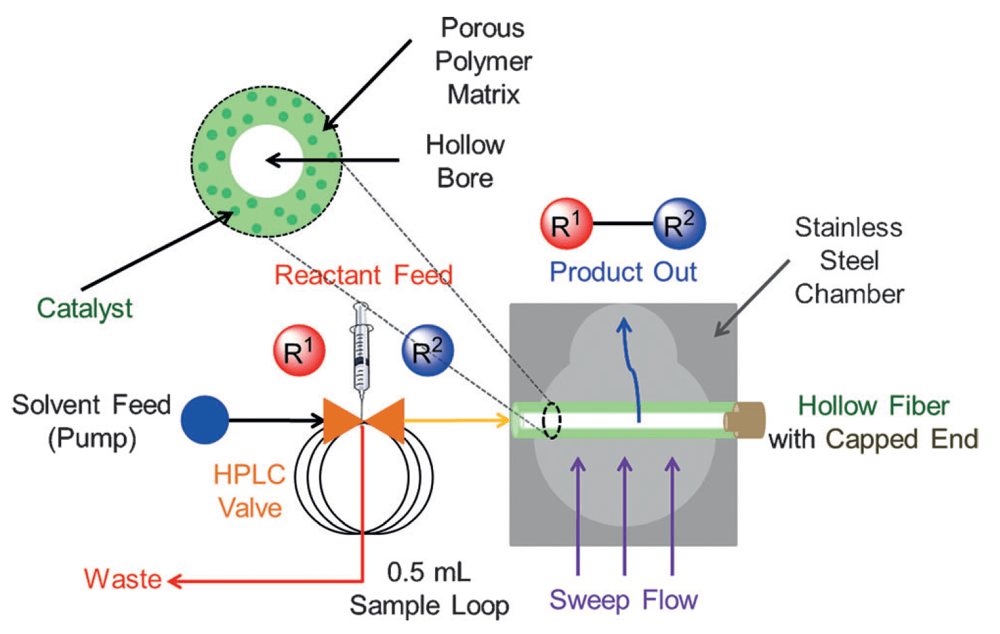
Composite Polymer/Oxide Hollow Fiber Contactors: Versatile and Scalable Flow Reactors
RESEARCH
-
11/2013
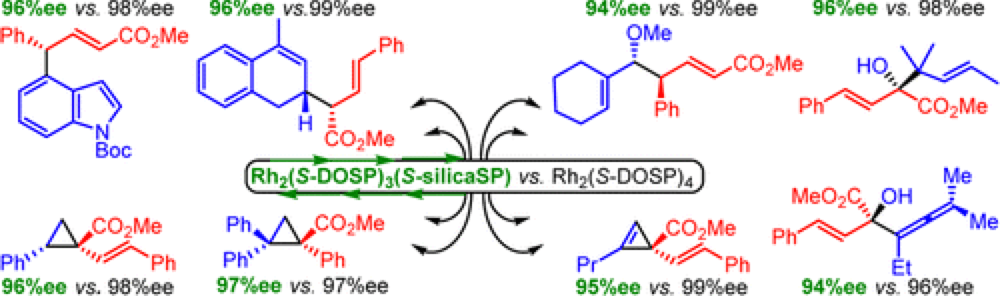
Silica-Immobilized Chiral Dirhodium(II) Catalyst for Enantioselective Carbenoid Reactions
RESEARCH