Silica-Immobilized Chiral Dirhodium(II) Catalyst for Enantioselective Carbenoid Reactions
Kathryn M. Chepiga, Yan Feng, Nicholas A. Brunelli, Christopher W. Jones and Huw M. L. Davies
Organic Letters,
2013, 15, (24), 6136-6139; 10.1021/ol403006r
11/2013
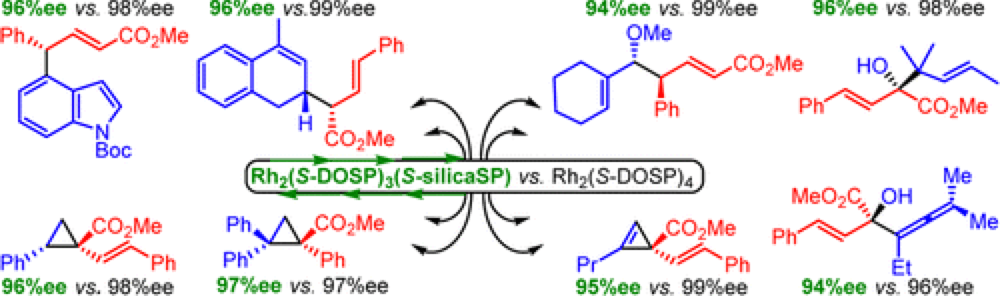
A silica-supported dirhodium(II) tetraprolinate catalyst was synthesized in four steps from l-proline and used in a range of enantioselective transformations of donor/acceptor carbenoids. These include cyclopropenation, cyclopropanation, tandem ylide formation/[2,3] sigmatropic rearrangement, and a variety of combined C–H functionalization/Cope rearrangement reactions.
The products of these transformations were obtained in yields and levels of enantioselectivity comparable to those obtained with its homogeneous counterpart, Rh2(S-DOSP)4. The silica-supported Rh2(S-DOSP)4 derivative was successfully recycled over five reactions.
Successful immobilization and application of the dirhodium catalyst paves the way for the development of new reactor systems for these highly reactive reagents for C–H Functionalization and a technique for the efficient reaction of new catalysts devised in the Center.