Structural basis of the Cope rearrangement and cyclization in hapalindole biogenesis
Sean A. Newmister, Shasha Li, Marc Garcia-Borràs, Jacob N. Sanders, Song Yang, Andrew N. Lowell, Fengan Yu, Janet L. Smith, Robert M. Williams, K. N. Houk and David H. Sherman
Nature Chemical Biology,
2018, 14, 345–351; DOI:10.1038/s41589-018-0003-x
03/2018
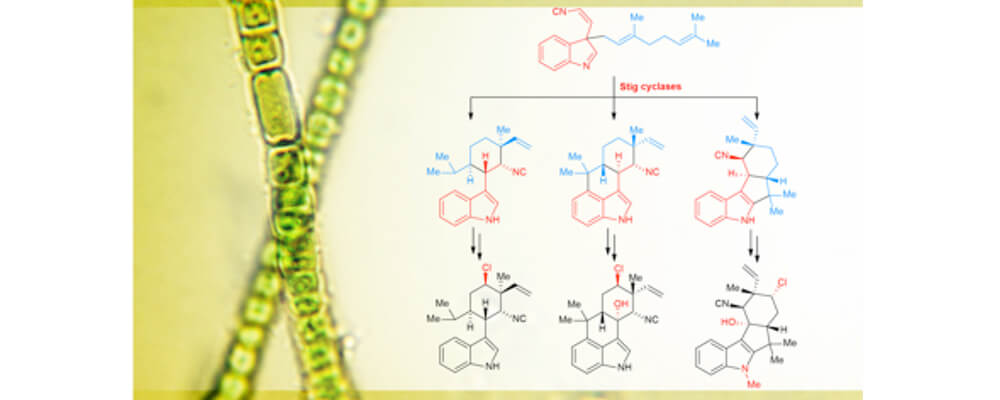
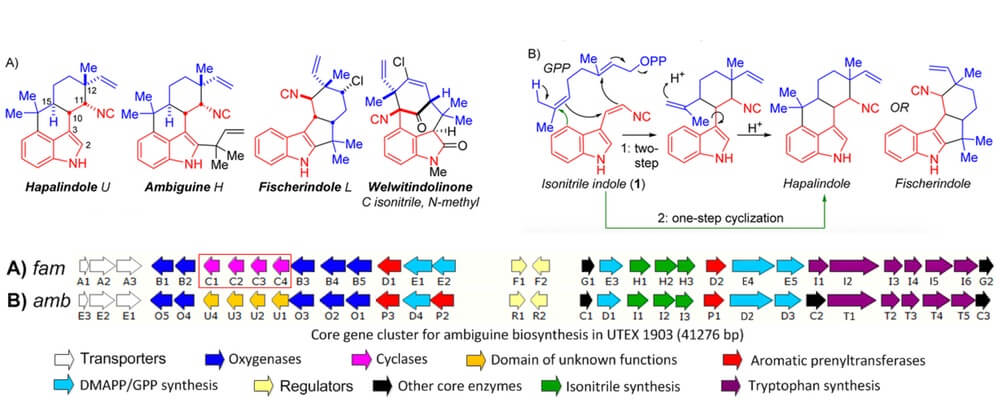
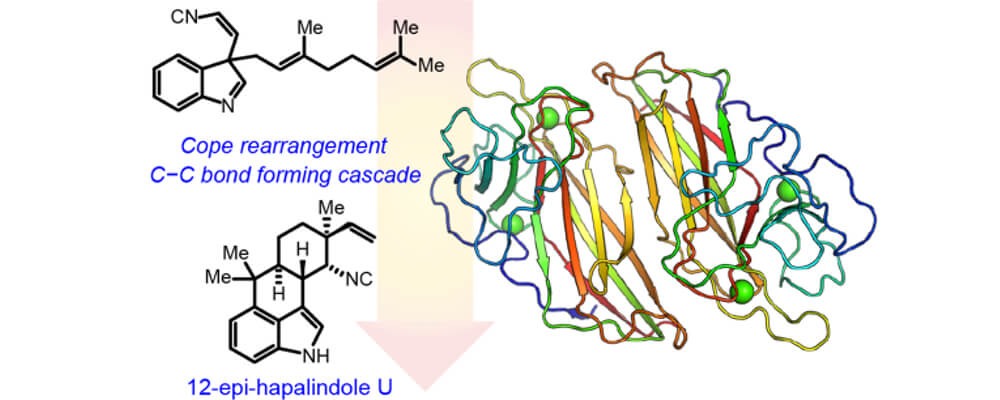
In this manuscript we report the first crystal structure of a Stig cyclase, HpiC1, which catalyzes the formation of 12-epi-hapalindole U in an unprecedented enzymatic transformation that involves three steps: a Cope rearrangement, 6-exo-trig cyclization, and electrophilic aromatic substitution which together set four stereocenters in the final product.
This remarkable enzymatic cascade occurs in several homologous Stig cyclases to generate the broad stereochemical and regiochemical diversity observed in the hapalindole family of alkaloids from a single common biosynthetic intermediate.