Enzymatic hydroxylation of an unactivated methylene C–H bond guided by molecular dynamics simulations
A. R. H. Narayan, G. Jiménez-Osés, P. Liu, S. Negretti, W. Zhao, M. M. Gilbert, R. O. Ramabhadran, Y.-F. Yang, L. R. Furan, Z. Li, L. M. Podust, J. Montgomery, K. N. Houk and D. H. Sherman
Nature Chemistry,
2015, 7, 653–660;10.1038/nchem.2285
06/2015
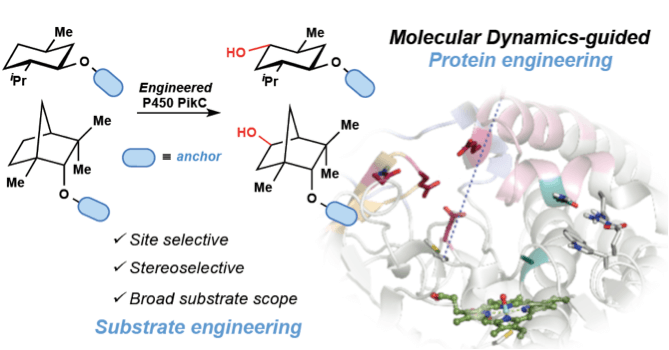
Profs. David H. Sherman and John Montgomery at the University of Michigan working together with Prof. Ken Houk at the University of California, Los Angeles and Prof. Larissa Podust at the University of California, San Diego have engineered an enzyme capable of C-H bond hydroxylation with exquisite site and stereoselectivity orthogonal to the selectivity trends demonstrated by traditional small molecule catalysts.
Pairing the computational expertise of the Houk group and the organic synthesis skills of the Montgomery lab with a natural product biosynthetic enzyme, P450 PikC, studied extensively by the Sherman group led to the development of a new tool for selective C–H bond hydroxylation. Building upon the basic mechanistic understanding of how PikC binds to its natural 12- and 14-membered macrolide substrates, the collaborators hypothesized that unnatural substrates could be designed to mimic the natural binding interaction. Initial studies between the Sherman and Montgomery labs validated this theory by synthesizing unnatural “anchoring groups” appended to various substrates and successfully hydroxylating these substrates with PikC. The novel approach employed by the team for protein and substrate engineering relied on computations carried out by the Houk group to guide mutations to the protein, P450 PikC, and also to predict which substrates would be efficiently processed by the enzyme.
These efforts culminated in a rationally designed PikC variant with enhanced catalytic activity on a wider range of unnatural substrates in comparison to the wild type enzyme. Further, a computational model for site selectivity was developed to predict the level of selectivity and favored site of oxidation on a particular substrate.
Currently, the interdisciplinary team is applying the combined computational/experimental approach to further expand the substrate scope of PikC to new classes of molecules and to continue to improve the catalytic efficiency of PikC through protein engineering.